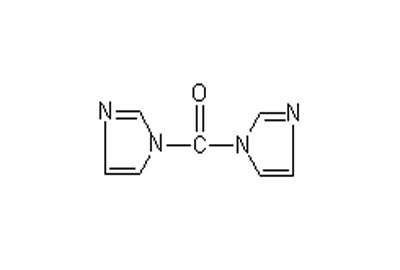
CDI reagent (N,N'-Carbonyldiimidazole)
| Name | CDI (N,N'-Carbonyldiimidazole) |
| CAS | 530-62-1 |
| MW | C7H6N4O |
| Purity % (HPLC) | 98.5% |
| Type | Peptide Coupling Reagent |
| Inventory | In stock |
| Price | 50g / $ 60.0 |
| Deliver | FedEx / DHL / EMS |

Omizzur Biotech Online Inquiry Form
Notice:
1.Omizzur follows strict guidelines on the use of private information.
2.For bulk inquiry or custom packaging: [email protected]
3.Our customer service staff will contact you in 1 hour. Please check your email

General Description
N. N-Carbodiimidazole (CDI) is mainly used as formyl transfer reagent and carboxylic acid activation reagent in organic synthesis. Because of its high reactivity, low cost and simple post-treatment, the reagent has higher application value than DCC and EDC.
The carbonyl group in CDI molecule is activated by imidazole and has very high formylation activity. When the reagent encounters hydroxyl group or amino group with active hydrogen, it can lose two imidazoles to form carbonyl derivatives at the same time according to the reaction conditions, and it can also lose one imidazole to form N-imidazole formyl ester or N-imidazole formamide. The resulting N-imidazole formyl compound can undergo further reaction to displace another imidazole to form a carbamide derivative with asymmetric structure.
Product Data Sheet
| Product Name | CDI (N,N'-Carbonyldiimidazole) |
| CAS No. | 530-62-1 |
| Molecular Formula | C7H6N4O |
| Molecular Weight | 162.15 |
| Appearance | White to off-white powder |
| Purity (HPLC) | 98% min. |
| Melting Point | 115~122 oC (dec.) |
| TLC Analysis | One spot |
| 300 MHz 1H | Consistent |
| Loss on Drying | < 0.5% |
Shipping & Storage
Storage :Keep in cool and dry place; easy to decompose when meeting with water.
Packing :100g / bottle; 500g / bottle; 1kg / bag; 2kg / bag; 20kg / barrel
Ordering Guide:
1. Staab, H. A.; Wendel, K. Org. Synth., 1968, 48, 44.
2. Kishikawa, K.; Nakahara, S.; Nishikawa, Y.; Kohmoto, S.;Yamamoto, M. J. Am. Chem. Soc., 2005, 127, 2565.
3. Nyangulu, J. M.; Galka, M. M.; Jadhav, A.; Gai, Y.; Graham,C. M.; Nelson, K. M.; Cutler, A. J.; Taylor, D. C.; Banowetz,G. M.; Abrams, S. R. J. Am. Chem. Soc., 2005, 127, 1662.
Customer also viewed
 TBTU
TBTU
CAS:125700-67-6
 HOBT Reagent (anhydrous)
HOBT Reagent (anhydrous)
CAS:2592-95-2
 HATU
HATU
CAS:148893-10-1
 HBTU
HBTU
CAS:94790-37-1
Enquiry & Ordering Form
Method 1
Simply email to [email protected] ,you will get a fast response within 1 hour.
Method 2
Submit the simple form to get the latest quotation. Notice:Omizzur follows strict guidelines on the use of private information.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap