Peptide Impurity Synthesis - Omizzur

What is Peptide?
Peptides are a type of compound formed by connecting amino acids through peptide bonds. They are usually composed of 10-100 amino acids. They are connected in the same way as proteins and have a MW weight less than 10,000 Da.
In recent years, with the development and maturity of peptide synthesis, peptide drugs have become one of the hot spots in drug R&D. Due to their wide range of indications, high safety and great efficacy, they have been widely used in tumors, cardiovascular and cerebrovascular, hepatitis,and prevention, diagnosis and treatment of diseases such as diabetes and AIDS. Peptides have broad R&D prospects.
Peptides and impurities can be quickly synthesized in the labs. Scientists can easily find their chemical properties because their simple structure. The time required for peptides from clinical trials to obtain FDA approval in the United States is much shorter than for small molecule and protein drugs. The probability of peptides passing clinical trials is twice that of small molecule drugs.
Preparation Method of Peptide
At present, the synthesis of peptides is mainly divided into chemical and biological methods. For chemical methods, we can use solid-phase methods and liquid-phase methods. More than 90% of peptide drugs currently on the market can be prepared by these two methods.
Peptide drugs are mainly composed of amino acids and their derivatives. It can be obtained through the gradual connection of amino acids or the connection of multiple peptide chain fragments through a more complex process. We may use a variety of methods when producing drugs.
For example, in solid-phase synthesis, liquid-phase synthesis may also be used. Solid-phase methods may also be used to make some fragments in liquid-phase . Therefore, there are many methods for synthesizing peptides now.
The biosynthetic methods of peptides include fermentation method, enzymatic method, and genetic engineering recombination method. Each of these methods has advantages and disadvantages. We will discuss this in another article.
To study the impurities in peptide synthesis, in fact, the easiest thing to think of is impurities missing from the process, because in solid-phase synthesis, every step cannot be 100% condensation, and there will inevitably be a lack of certain amino acids, and the peptide chain itself may be broken ,some structures may be changed. These are very important in the analysis method.
Another is degradation impurities. For example, some amino acids are prone to hydrolysis, oxidation, or even polymerization. We need to consider these issues.
Currently, when applying for new peptide products, plus the two types of impurities above, ICHQ3 also requires the study and control of the remaining solvent residues, elemental impurities, and genotoxic impurities etc ,.
Solid phase synthesis of peptides has been widely used. During the synthesis and storage of peptides, various peptide impurities may be occur. It including loss of amino acids, insertion of amino acids, degradation products, D-amino acids etc. Many impurities not only have no great effects, but also have bad side effects. The EP requires analysis of impurities with a content exceeding 0.5%. Detection toxic and side effects is needed.
Our team can provide thoughtful service and support for peptide impurity synthesis. Most products can be delivered within 2-3 weeks, in quantities of 50-500mg, and with purity of 90-95%. Also we provides all test reports (HPLC,MS,COA). If you have any questions, you can contact our customer service team directly.
>> Salmon Calcitonin Impurities
>> Liraglutide Impurities
Customer Service Center
 Need Quote Request :
Need Quote Request :
* Please mail us your product sequences / structural formula, CAS (if any), Omizzur customer services will get in touch with you within 1 hour.
FAQS: 2 kinds of Impurity Control in Peptide Synthesis
Impurity control in peptide synthesis actually needs to be considered first from the synthesis strategy. The first step is to consider its amino acids. We need to find a correct synthesis ways.
Regarding the control of starting materials, the first thing we think about is amino acids, because impurities in amino acids may be go to the peptide products, so how to control them?
There are many types of amino acids used in synthesis. Here is an example, such as double-protected lysine. L-form lysine, which may contain D-form. In addition, during the preparation process of this double-protected amino acid, it may lose a protecting group, form a dimer, or beta-alanine, or even exchange the positions of the two protecting agents, or both protective bases all fell off. These all may be brought into the product, so these types of impurities need to be analyzed.
This is just 1 amino acid as an example, but in synthesis process, more than 10 or 20 protected amino acids are often used, so the control of starting materials requires a lot of QC work for the analysis. This is an important step for all of us.
Control during synthesis. In addition to controlling impurities from the source amino acids, peptide synthesis is also more important to control during the process. This step is also very important. How to ensure that the condensation of every amino acid is complete during the synthesis process?
The most commonly used method is the ninhydrin detection method. Its is very sensitive. As a qualitative in-process control method, it is currently used by everyone. There are other ways, such as TNBS and tetrachlorobenzoquinone method. If you want better control, you can use the LC-MS . Each step of the condensation reaction, including the deprotection fmoc group. It can be more accurately controlled.
Peptide Impurity Control Method
The control method for impurities is mainly carried out from 3 aspects.
1: control of synthesis. Good processes can reduce the generation of impurities, control the content of impurities at a lower level;
2: control purification. Use effective separation methods to remove or reduce them;
3: Suitable storage method can reduce the generation of impurities. for details you can mail us for support.
Review of Peptide Drugs
The review of chemically synthesized peptide drugs is just as chemical drugs. In pharmaceutical R&D, it is basically similar to small molecule synthetic drugs. The main research includes the study of the preparation of raw materials, structural confirmation, formulation, quality and stability research, etc. It is all the same as other chemical drugs. Peptides may have some characteristics of their own.
Whether it is a generic drug or an innovative peptide drug, the application materials are now required to be submitted in CTD format . The core content of the CTD is actually the main part of the DMF file. Module 2 is the quality review, of which 2.3S3.2 is the impurity research in the synthesis process. It requires all impurities and their analysis to be listed, and it must be controled. Solvent residue is also an important content to be studied.
DMF file module 3 puts forward very specific requirements for the research on impurities. When making DMF files, a lot of energy is spent on the research and control of impurities in 3.2S3.2. Now, in accordance with the requirements of CDE review, the requirements for the source of impurities, the quality control of starting materials, have been greatly increased.
Because there are many impurities in the peptide synthesis process, applying for a peptide product requires a lot of research work. Impurity R&D is very expensive and time-consuming wrok, but this part of the work best reflects the level of peptide R&D. If your team do this well, your chances of passing the review will be much greater. In recent years, as review requirements have increased, we have also improved our capabilities in peptide synthesis process development, impurity control, and product analysis.
Due to the characteristics of solid-phase peptide synthesis, it is difficult to achieve a total yield of 99%. It is possible to achieve a purity of more than 99% through purification. As far as synthesis is concerned, it is not enough for the condensation reaction in each step to reach 99%. It is best to be close to 100%.
The purity of peptides can be improved through process control, including purification and analytical ways. There are many manufacturers producing peptides now. The core is the quality of the product, that is, the level of impurity control, the other is the production cost.
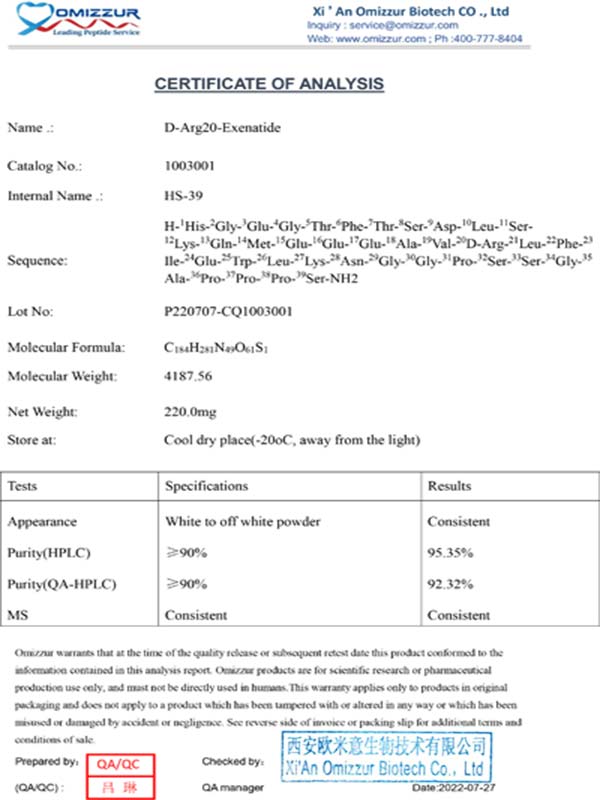
COA of Peptide Impurity Cases:
6 Peptide Impurities Which Appear During Peptide Synthesis & Storage :
1. Loss and incorrect insertion of amino acids
In solid-phase synthesis of peptides, mistakenly removing the protective groups of amino acids or introducing wrong activated amino acids can reduce the efficiency of reactions. This can lead to the loss of amino acids in the peptide chain.
During shipping and storage, peptides also produce a small amount of degradation products. The loss of amino acids at the N or C end of a peptide is also said as an amino acids impurity.
On the other hand, in peptide synthesis, excessive amino acid raw materials are usually added to ensure MAX synthesis efficiency. If its not been removed after the reaction, additional amino acids may be inserted into the peptide sequence.
2. Protective group residues
In solid-phase synthesis of peptides, sometimes the protecting group cannot be completely removed (amino group , side chain protection, etc. It will cause the protecting group remain in the target peptide.
3. Oxidation / reduction
Certain amino acid residues are easy to oxidation/reduction reactions during solid-phase synthesis. Prolonged exposure to light or exposure to air during storage of histidine and lysine amino acids may lead to different impurities. We often see this situation during normal research.
4. Diastereomers
This refers to the racemization of amino acids. Even a small amount of isomeric impurities can greatly affect the function. This situation is common in drug R&D, and we need to pay more attention to this issue. You can contact our customer service for help.
5. Side chain / terminal
Side chain impurities can be mainly divided into 2: impurities introduced by side chain protecting groups and impurities introduced by amino acid side chain itself with reactivity. Such as the analysis of the side chains of two basic amino acids, asparagine and glutamine.
6. Peptide aggregates
This can be divided into two types, covalent and non-covalent, and the degree of its formation depends on various environmental factors. Covalent aggregates are usually formed by two monomers through amide bonds and disulfide bonds. Check our konwledge center you can learn more about it.
Detection Methods for Substances Related to Peptide Drugs
1. Reversed-phase high-performance liquid chromatography (RP-HPLC)
HPLC method has become the most commonly used method in drug quality analysis. It is sensitive, accurate and fast.
Peptides are made of amino acids connected through peptide bonds and have a certain degree of hydrophobicity. On a C18 column It has certain retention and can be effectively separated in RP-HPLC.
Nowadays, RP-HPLC method is mostly used for the analysis of related substances of peptide and impurities. Since the substances that may be contained in synthetic peptide drugs are complex, gradient elution methods are often used, and isocratic elution is used in very few cases. We need study according to the actual situation.
2. Capillary electrophoresis (CE)
In addition to using traditional HPLC to analyze related substances, capillary electrophoresis (CE) also has great advantages in the analysis of peptide drugs. CE has the advantages of both high-voltage electrophoresis and HPLC. It is different from the mechanism of HPLC. There are multiple separation modes. CE has fast analysis speed, high resolution, and low sample consumption. CE is easy operation and low consumption.
CE is used in pharmacopoeias of various countries. USP, BP, and Chp all include CE method, and introduce its definition, classification, basic principles, instrument structure and operating parameters in detail.
In USP and BP, CE has been used in the quality standards of multiple drug varieties. Ulrike Holzgrabe reviewed the application of CE in EP, JP, and USP, and its application in impurity analysis, especially in the analysis of impurities in peptide and protein drugs. At present, CE has great advantages and been used as a detection method in the quality control of peptide drugs.
In EP 7.0, CE is used for analysis of glutathione related substances. In the future, with the improvement of the CE itself and the development of instruments to overcome its shortcomings of low reproducibility and precision, CE will become a powerful tool for the analysis of peptide drugs.
3.HPLC-MS method
HPLC-MS has now become an important means of separating and identifying various compounds. MS method is a powerful structural analysis tool that can provide more information for structure. HPLC-MS method not only has strong specificity , but also has extremely high sensitivity.
When using HPLC-MS to analyze substances related to peptide drugs, the first-level or even multi-level MS information of the impurity can be given without obtaining pure impurities. We can obtaining the molecular weight and sequence information, and identifying the related peptides. It provide basis for structural analysis.
Our team checked many literature and found that there are currently many reports on the use of HPLC-MS to study chemical drugs and related impurities, but there are few reports on research on substances related to peptide drugs. Now HPLC-ESI-MS/MS is widely used in R&D such as peptides and proteins.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap