Peptide Characterization APIs (3): Content determination
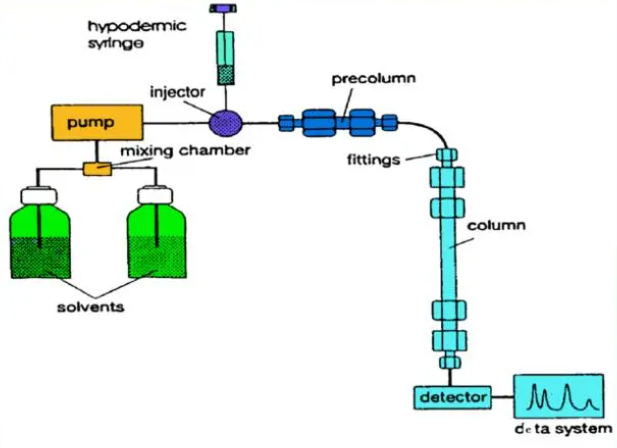
Content (potency) determination is one of the main indicators for evaluating the quality of peptide raw materials. In the pharmacopoeia, comparative chromatography is usually used for determination (sample weighing must be carried out under temperature and humidity control conditions), with the determined chemical reference substance as the standard, but there must be sufficient quantitative reference standards (which themselves must undergo full analysis). Indicators are usually expressed as acetic acid free or anhydrous peptides. The allowable limit for this type of detection is usually asymmetric, with an upper limit of 100%+allowable test repeatability (usually 2.0%) and a lower limit of 100% - (allowable test repeatability+maximum allowable impurity level). The prerequisite for using comparative chromatography is that there must be sufficient quantitative reference standards.
Amino acid composition analysis method
The determination of peptide content can also be easily determined by amino acid composition analysis results, and the total mass of the sample can be obtained by calculating the molar content of each detected amino acid. The disadvantage of this method is that some amino acids such as tryptophan may be destroyed during the peptide hydrolysis process, resulting in inaccurate detection results. Other methods include nitrogen determination through elemental analysis, Kjeldahl nitrogen analysis, and the use of nitrogen specific detectors in HPLC detection, but these methods have no specificity for amino acid derived nitrogen and are prone to systematic errors
Other tests
In addition to the important quality attributes mentioned above, quality control also needs to consider whether other attributes of the product need to be tested, such as general attributes, including appearance, color, solubility, clarity, acidity, visible foreign objects, optical rotation, and quality balance. These attributes are general requirements for quality control of peptide raw materials, and can also demonstrate consistency between product batches. The clarity, color, and pH of the solution are important indicators for quality control of raw materials, and these two checks should usually be conducted, especially for peptide raw materials for injection. In addition, most peptides are administered by injection or oral administration, so there are requirements for microbial and endotoxin testing.
From the perspective of quality balance, the detection of peptide raw materials should control the sum of their peptide, main component ions, and moisture content, generally requiring a control of 100% ± 5% to ensure the absence of a large amount of inorganic salts or other unknown impurities.
The importance of reliable quality control indicators
For the production of peptide raw materials, establishing comprehensive and reliable quality control indicators is a necessary prerequisite for establishing repeatable production processes. The above identification, testing (purity), content determination, and general attribute testing are necessary components of the full inspection of peptide raw materials. According to the different production processes of different peptides, the analysis and characterization content of peptide raw materials can be adjusted appropriately. Peptide raw materials can only be released for use after passing all predetermined quality tests
>> Ask quote for custom peptide synthesis
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap