Peptide Characterization APIs(2): Purity
The purity test is mainly used to limit the impurities in the related substances and test the purity and integrity of the peptide. In addition to the target peptide, other peptides can be regarded as impurities, mainly including process-related impurities and degradation products, polymers, residual solvents, main component ions, moisture, etc. that are similar in structure to the target peptide.
Process-related impurities refer to related impurities produced during the synthesis and production of peptides. For example, in the process of peptide synthesis and production, in order to prevent unwanted side chain reactions, many side chain protecting groups will be introduced, and then the side chain protecting groups will be removed as needed. Since these chemical reactions are not 100% complete, eventually side chain protecting groups may still attach to the target peptide, resulting in the appearance of peptide-associated species. In addition, regardless of the production process, peptide degradation products may be generated due to chemical instability. Peptides generally produce degradation products through different mechanisms such as hydrolysis, oxidation, and racemization.
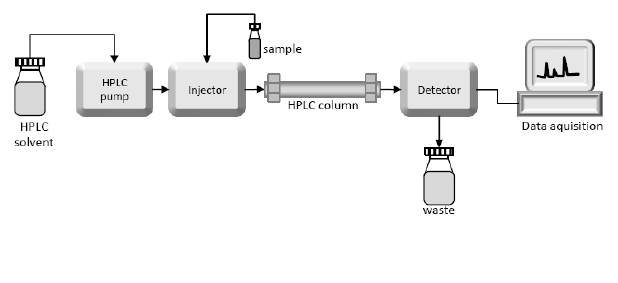
HPLC-Peptide Purity Analysis
High-performance liquid chromatography (HPLC) is the main method for the identification, separation and quantification of peptides and their related impurities. Whether the impurities can be detected, separated and identified is an important factor to measure the quality of the method used. One of the basis. For peptides, the elution performance of different columns varies greatly. At present, there are many peptide analysis columns on the market. If they are selected properly, the separation of impurities in peptide APIs can be significantly improved. Peptide APIs in Pharmacopoeia generally have specifications and impurity limit requirements. Impurities may or may not be specified, depending on the amount they are present.
Polymer detection
Polymer is an impurity produced by surface adsorption, aggregation or precipitation due to the physical instability of the product, and the content of monomer and polymer is generally detected by size exclusion chromatography. Elemental and organic reagent impurities often come from leaching of materials, reagents and equipment used in product synthesis and purification processes, etc.
Peptide principal component ion detection
For the detection of peptide main component ions (such as acetate, hydrochloride, and trifluoroacetate) and moisture, reversed-phase chromatography and ion-exchange chromatography are the most commonly used methods. Reversed-phase chromatography is mainly used to detect trifluoroacetate and acetate, but its detection range is narrow, and there are many and obvious impurity peaks, so the separation effect may not be good. However, the reversed-phase chromatographic column can tolerate organic solvents, so a small amount of organic solvent can be added to dissolve the water-insoluble polypeptide samples, and then detected by reversed-phase chromatography. In contrast, ion exchange chromatography generally cannot use organic solvents, otherwise it will damage the column. Of course, in some cases special ion-exchange columns can be used instead.
Determination of moisture:
Moisture is mostly determined by Karl Fischer titration. Moisture is not only an important indicator for product release, but also a commonly used inspection indicator in product stability testing.
Karl Fischer titration can be divided into coulometric titration and potentiometric titration according to the principle. Among them, coulometric titration has high sensitivity and is suitable for trace moisture detection. It takes a long time to detect moisture with high content. Different moisture determination methods can be selected according to the hydrophilicity and hydrophobicity of the peptide API. The Pharmacopoeia also stipulates that the water should not exceed 3% to 14% based on the hydrophilicity and hydrophobicity of the peptide.
Fast link
>> Omizzur custom peptide synthesis
>> Peptide impurity synthesis services
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap