Chiral synthesis of unnatural amino acids (3)
Unnatural amino acids are a kind of non protein optical amino acids that cannot be directly obtained in nature. This kind of amino acids can not be obtained by fermentation, but can only be synthesized by chemical synthesis or engineering bacteria fermentation to produce enzymes for enzyme catalytic synthesis
In this general formula, R group represents various substituents such as alkyl and aromatic groups, forming chiral compounds with special chiral centers. The carboxyl group in this structure can be reduced and esterified in different ways to form different chiral compounds. Amino groups can also be protected by various groups such as tert butoxycarbonyl (BOC), benzyloxycarbonyl (CBZ) and Wat methoxycarbonyl (Fmoc) for example Fmoc-Pen(Acm)-OH ,Fmoc-Pen(Trt)-OH. forming chiral compounds into the side chain of chiral drugs. Most of the chiral centers of traditional chiral drugs are achieved by asymmetric catalysis or resolution. This method is not efficient and chiral purity, which greatly limits the development of chiral drugs. Therefore, it is of great significance to solve the synthesis of unnatural amino acids and the development of chiral drugs
Unnatural amino acids:
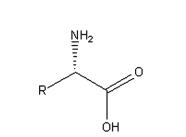
Properties of unnatural amino acids
The structure of unnatural amino acids is similar to that of natural amino acids, but the chiral direction is different. Most of them have good water solubility and high melting point, which are generally above 200 ℃. The free state is relatively stable, and the form of internal salt exists. Non natural amino acids have amino and carboxyl groups, which are relatively active, and have great natural advantages in the later group transformation
The next chapter will explain examples of the functions of unnatural amino acids
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap