Fmoc-OSu Synthesis
Fmoc-OSu technology field:
The invention is a synthetic method of Fmoc-OSu, which is mainly used in biochemical pharmacy and as an amino acid protector. There is a demand of 300~600t in the international market every year.
Background technology
Polypeptides are bioactive substances related to various cell functions in life. It is a kind of compound whose molecular structure is between amino acids and proteins. It is successfully synthesized by peptide bonds with a variety of amino acids in a certain arrangement. The total synthesis of peptides has important application value. Through the total synthesis of peptides, the structure of peptides can be verified, new peptides can be designed to study the relationship between structure and function, and provide important information for the mechanism of peptide biosynthesis reaction: the establishment of model enzymes and new drug peptides. Peptide synthesis has become one of the most active fields of biochemical science and technology. At present, polypeptides are mainly synthesized by solid-phase and liquid-phase methods.
About Fmoc-OSu
In the process of peptide synthesis, the active group of amino acid must be protected first, and then the protective group can be removed after the reaction is completed. In 1978, Chang meienlofer and Atherton adopted Fmoc-OSu as the amino protecting group The Fmoc group is very stable to acid and is removed with piperidine dichloromethane or piperidine dimethylformamide. Now Fmoc solid-phase peptide synthesis method has been widely used. Fmoc-OSu is the most effective reagent to provide Fmoc groups.
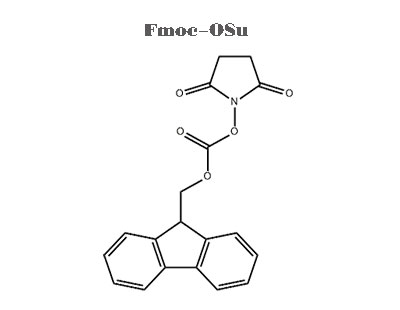
Problems in Fmoc-OSu synthesis
Fmoc-OSu is generally synthesized by reacting Fmoc chloroformate with succinimide in dioxane solvent in the presence of organic bases such as triethylamine. The Fmoc OSU synthesized by this method has high solubility in dioxane solvent, and dioxane and triethylamine can not be completely recovered, which pollutes the environment. Moreover, the existence of triethylamine and other organic bases makes the product easy to decompose, which makes the product difficult to refine and purify, with low yield and purity, so it is not suitable to be directly used in industrial production.
Advantages: the synthetic process is simple, the reaction conditions are mild, the non-toxic and harmless ethyl acetate solvent is used, and it can be completely recycled, which is easy for industrial production. The synthesized product is easy to separate, with high yield and purity. It can be used for the preparation of protective amino acids in peptide synthesis without refining.
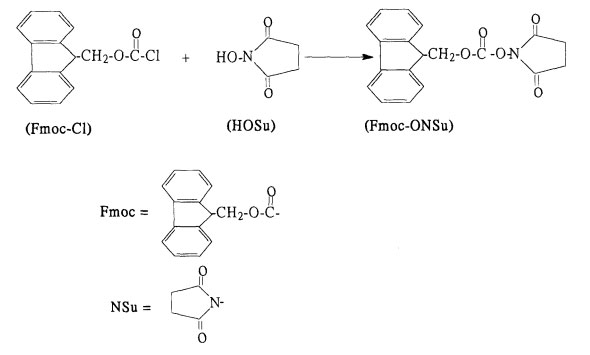
Fmoc-OSu synthesis steps
Add the aqueous solution of succinimide to the ethyl acetate solution of Fmoc-Cl, add inorganic base in batches, stir, react, filter, wash and dry to obtain the product. The crude product after the reaction can be directly used for the synthesis of protective amino acids, and the pure product Fmoc-OSu can also be prepared. Fmoc-CL in the filtrate can be further recycled
Matters needing attention:
1. The suitable concentration of Fmoc-CL in ethyl acetate is 10-30%
2. The suitable concentration of hosu in water is 5-15%
3. The ratio of the appropriate amount of Fmoc Cl and hosu is 1:0.8~1.25
4. The more suitable reaction temperature is 15~35 ℃, and the more suitable reaction time is 2-2.5 hours
For more notes, contact omizzur biotech
Advantages of the new synthesis method of Fmoc-OSu:
1. The reaction is in the two-phase interface, and the finished product precipitates in the form of solid, which is easy to separate.
2. Unreacted Fmoc Cl and a small amount of by-products in the filtrate are easily soluble in ethyl acetate. Fmoc-OSu with high purity can be used for the preparation of protective amino acids without refining.
3. Fmoc-CL can be used repeatedly, and the product yield is high
4. By adding sodium carbonate or sodium bicarbonate in batches, the organic layer is basically neutral, which reduces the production of 9-fluorene methanol and improves the purity of the product.
5. This method uses non-toxic and harmless ethyl acetate solvent, which can be completely recycled. The reaction only produces a small amount of neutral brine, which is easy to treat and is conducive to industrial production.
-----
Omizzur biotech supplies a large number of Fmoc-OSu and CBZ-OSu, protected amino acids, peptide synthesis and other products. main advantage markets are in Europe, the United States, India etc. The next chapter will demonstrate the example of synthesizing Fmoc-OSu.
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap