Synthesis of exenatide
Abstract
Objective:To develop a high efficacy and cost-effective method for the synthesis of exenatide to meet the requirement of a scale production. Methods: Thirty-nine amino acids in exenatide were divided into 13 small protected peptide fragments based on N-C extension strategy, the first to 12th fragments were synthesized by a liquid-phase method, respectively while the 13th fragment was synthesized by a solid-phase method and then the first to 12th fragments were in turn connected into. Fmoc-protective fragment to obtain exenatide resin, which was cleavaged to obtain crude exenatide. Finally, exenatide was obtained by reverse phase HPLC purification and lyophilization.Results: The structure of the synthesized exenatide was verified to be correct by mass spectrometry characterization and its purity was >98% by HPLC. Conclusion: The synthesis efficiency can be improved and the accumulation of impurities and the diffculty of purification can be reduced by this method, which is suitable for the large-scale production of exenatide.
Introduction to exenatide and its medical effects
Exenatide is an active peptide containing 39 amino acids with the amino acid sequence:H2N-His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-CONH2which is also a type of glucagon GLP-1 analogs.Studies have confirmed exenatide has the effects of blood glucose control, weight reduction, cardiovascular protection and inhibition of MCF-7 cell proliferation in breast cancer.Exenatide injection can simulate the hormone regulating insulin in the body and play a role in promoting insulin secretion, increasing insulin sensitivity and improving the function of islet cells, so as to control blood sugar.Exenatide can slow down food absorption by slowing gastrointestinal peristalsis, and act on the hunger center of the brain to suppress appetite and reduce energy intake, thereby reducing weight.Exenatide also has cardiovascular protective effects, improving endothelial cell function, promoting sodium excretion, improving ischemic myocardial injury and recovery of cardiac function, and reducing risk factors for cardiovascular risk. Studies have shown that selective GLP-1 receptors exist on breast cancer MCF-7 cells, and exenatide can inhibit the proliferation of breast cancer MCF-7 cells.
At present, the preparation method of exenatide is mainly the traditional classical solid phase peptide synthesis method, and the raw materials used in the efficacy evaluation of exenatide are synthesized by this method. The synthesis process is simple and can be automated, but the synthesis cycle is long, the feeding amount is low, the synthesis efficiency is low, the purification cost is high, and it is not easy to scale.Using threonine, oxazole derivatives of serine that are structurally similar to proline, these pseudoprolines prevent polypeptide aggregation and the formation of beta folds. In the solid-phase synthesis of exenatide, Rink Amide MBHA resin and Fmoc-Rink Amide PEGMatrix resin were used as starting resins, respectively. Full protective exenatide was synthesized by Fmoc solid synthesis by coupling amino acids and proline-like pseudodi peptide fragments with protective groups one by one. The product was cleaved and purified.This method greatly improves the synthesis scale and the product purity, but the cost of raw materials used in the reaction process is still high.Some biologists divided exenatide into six fragments for synthesis. First, the fragments were assembled and cut into the crude exenatide protected by Fmoc, and then the pure exenatide was obtained by deprotection and purification. This method combined the advantages of solid phase method and liquid phase method, but the feeding amount was small, only 0.66 mmol, and the amino acid sequence contained in the liquid phase synthesized fragment was long, and the purity of the synthesized fragment was difficult to control. In order to meet the needs of large-scale production, developing a high efficiency and low cost synthesis method of exenatide has become an urgent technical problem in this field.
The synthesis of exenatide by solid-phase combination method overcomes the shortcomings of traditional solid-phase polypeptide synthesis, such as low efficiency, low product content and high purification cost, and improves the synthesis efficiency, reduces the purification cost, and is easy to process and scale.
Exenatide product obtained
The cutting solution consisting of 83% trifluoroacetic acid, 5% phenol, 4% anisole, 3% water and 5% triisopropyl silane was stirred at room temperature for 2 h, filtered and precipitated with cold ether to obtain exenatide crude. Finally, 14.13 g exenatide was obtained by reverse phase chromatography purification and freeze drying. The yield was 33.1% (calculated with 10.2mmol initial yield of Rink Amide-MBHA Resin, the theoretical yield of exenatide was 42.71g
Results
HPLC analysis(Figure 1 HPLC chromatogram of exenatide)
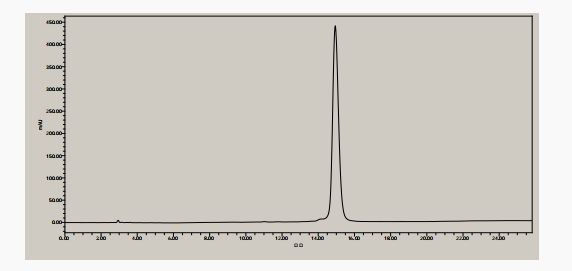
The purity of the synthesized exenatide was determined by liquid chromatograph. The liquid chromatogram was shown in Figure 1. The purity of Exenatide was greater than 98%, consistent with the retention time of the control product
tructural characterization of exenatide:(Figure 2. Mass spectrum of exenatide)
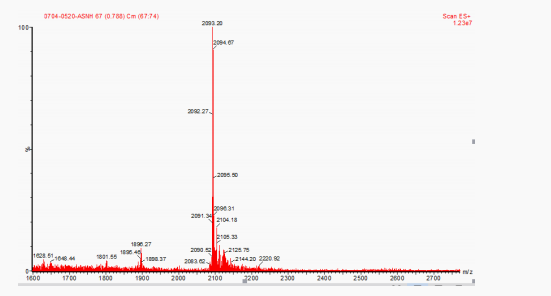
The structure of the synthesized product was characterized by mass spectrometer, and the results were shown in Figure 2. The molecular weight of Exenatide was 4 186.6. It can be seen from Figure 2 that [M+2]2 peaks, 2 093.20, were consistent with the molecular ion peak of Exenatide, which proved that the molecular weight of the synthesized Exenatide was correct.
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap