Pbf Protecting Group
In peptide synthesis, it is not only necessary to protect the amino and carboxyl groups that do not participate in the formation of amide bonds, but also to protect the active genes on the amino acid side chain.
After the completion of the reaction, the protective genes can be removed to successfully synthesize peptides with a specific amino acid sequence, among which the sulfhydryl group of cysteine and the amino group of lysine need to be protected without exception.
The carboxyl groups of glutamate and aspartate need protection. Other groups in amino acids can sometimes be left unprotected.This article focuses on the protection of guanidine group of arginine side chain (Fmoc-Pbf-Arginine).
1. Protection of Guanidine Group in Arginine Side Chain
Arginine is an essential amino acid that can be produced naturally in the body. In life science research, the amino and guanidine groups of arginine must be protected in order to avoid the production of by-products.
Fmoc-pbf-arginine is an amino acid derivative obtained by using Fmoc(9-fluorene methoxycarbonyl) to protect the amino group and Pbf(2, 2, 4, 6, 7-pentamethylbenzofuran-5-sulfonyl) to protect the guanidine group.
Because of the strong water solubility of peptides not protected by arginine side chains, it is often difficult to isolate and purify the products. In order to weaken the basicity of guanidine and increase its solubility in organic solvents, guanidine protection is generally still used. Thionitro is one of the commonly used arginine side chain protection groups.
The Arg protected by Pbf is now commonly used by Omizzur Biotech . PBF protecting group refers to two,2,4,6,7 Pentamethyldihydrobenzofuran - 5 - sulfonyl group, it is a kind of common protecting groups.
What is Pbf protecting group: The pbf group protects the amino group in the amino acid or peptide from non-specific reactions with other functional groups. The pbf group can be removed by hydrolysis under acidic conditions, releasing the protected amino group.
The advantage of Pbf is that the removal method is simpler than that of nitro protection (95% of TFA can be removed). Arg(NO,) is easily prepared from arginine nitrated with fuming nitric acid plus fuming liu acid (or concentrated liu acid) under cold conditions or with KNO3 nitrated at 0℃ in
HF for 30 minutes.
The melting point of Arg(NO,) is 251 to 252℃. N-NO2 pair - series acid hydrolysis or alkali hydrolysis conditions are stable, but can be H2/Pd, electrolytic reduction, titanium trivalent reduction, HF(liquid) treatment to remove.
2. Structure And Properties of Fmoc-arg (pbf)-OH
Fmoc-arg (pbf)-OH is a compound used to synthesize organic molecules, which has three groups: fmoc, arg and pbf. Its structure contains an N-A-9-fluoroacyl-L-arginine residue and a P-phenylformyl fluoride (pbf) protection group, which forms an amide bond through the amino reaction of acyl chloride and arginine. This structure can be prepared by chemical synthesis.
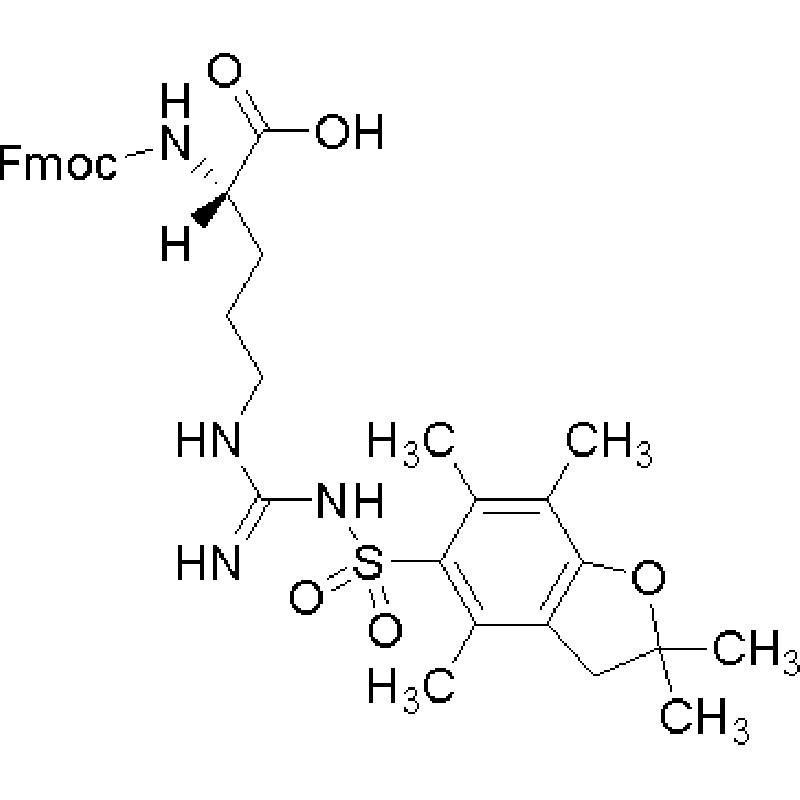
Fmoc-arg(pbf)-oh has certain biological activity, and it can interact with other biomolecules specifically. This is mainly due to the positive charge of the arginine residue in its structure. Arginine is an important amino acid that plays an important physiological function in cells.
Therefore, fmoc-arg(pbf)-oh can be used as a bioactive small molecule to participate in cell signaling, protein interaction and other biological processes.
3. Application of Fmoc-arg (pbf)-OH in Biomedical Field
In the field of biomedicine, fmoc-arg(pbf)-oh is widely used in many fields. First, it can be used as an antibacterial agent to fight bacterial infections.
residues can interact with the negatively charged surface of bacteria and destroy the cell membrane structure of bacteria, thus playing an antibacterial role. n addition, fmoc-arg(pbf)-oh can also be used as an antiviral agent to inhibit the replication and spread of the virus.
In addition to antibacterial and antiviral effects, fmoc-arg(pbf)-oh also has certain antitumor activity.
Studies have shown that arginine residues can be involved in the growth and proliferation of tumor cells through a variety of pathways, and therefore, fmoc-arg(pbf)-oh can be used as a potential anti-tumor drug. In addition, it can also be used for tumor labeling and tumor diagnosis.
In addition to its applications in the biomedical field, fmoc-arg(pbf)-oh can also be used in the field of materials science. Because the arginine residue in its structure has a variety of functional groups, it can interact specifically with the surface of the material,
Thereby changing the properties and properties of the material. This provides a new way for the modification and functionalization of materials.
fmoc-arg(pbf)-oh is a kind of chemical reagent with biological activity and application potential. It has specific interactions and a variety of biological activities, and can be applied to antibacterial, antiviral, anti-tumor and materials science fields.
With the in-depth study of the structure and properties of fmoc-arg(pbf)-oh, it is believed that its application fields will be more and more extensive, and make greater contributions to the development of biomedicine and materials science.
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap