Labeled Amino Acid of Peptide
With the development of proteomics and biomedicine, amino acid labeling of peptides and proteins has attracted wide attention and become a very important research direction.
By modifying amino acid labeled of peptides and proteins, the relationship between structure and function of proteins and protein-protein interaction can be studied, tagged molecules can be used to track and locate, and some active peptides and proteins can be modified.
There are many contents of peptide labeling and modification, which are widely used in the research of peptide drugs, peptide biology, peptide antibodies and peptide reagents. At present, biotin labeling, fluorescent labeling (FAM, FITC, etc.) and phosphorylation modification are widely used.
Fluorescent labeling technology is commonly used in biological experiments, Omizzur Biotech has been developed for a long time, and can provide a variety of fluorescent labeling peptides with mature technology. This paper focuses on the fluorescence labeling of peptides and amino acids.
1. What Is Fluorescent Labeling?
The compounds on which fluorescent labeling depends are called fluorescent substances. Fluorescent substances are compounds with conjugated double bond system chemical structure, which can be excited into an excited state when irradiated by ultraviolet light or blue or violet light, and fluorescence is emitted when the ground state is restored from the excited state.
Fluorescent labeling technology refers to the use of fluorescent material covalent binding or physical adsorption on a certain group of molecules to be studied, using its fluorescence characteristics to provide information about the object of study. Fluorescent labels are widely used in many research fields because of their advantages such as no radioactive contamination and easy operation.
2. The Role of Fluorescent Labeling
Fluorescent labeled substances are also widely used in the functional study of proteins and drug screening. The use of fluorescentially labeled peptides to detect the activity of target proteins has been applied to drug screening and drug development (e.g., various kinases, phosphatases, peptidases, etc.) of protein targets for disease treatment by developing high-throughput active screening methods.
Therefore, fluorescent modification of peptides is also an important content in the field of peptide synthesis.
3. Fluorescent Labeled Sites
Fluorescent labeled substances can be directly connected to the N terminal of the peptide (such as the connection of fluorescein isothiocyanate, which is usually inserted directly into the N terminal of the peptide and fluorescein isothiocyanate or β-alanine). Either a side chain connection with lysine (or cysteine) at the C-terminal, or a link in another location that can be connected.
4. Examples of Fluorescent Labeling
4.1 FITC modification
Fluorescein isothiocyanate (FITC) has a relatively high activity, and in general, it is easier to introduce this fluorophore than other fluoresceins in the solid phase synthesis process, and no activation reagent is required during the reaction.
Fitc-modified peptides usually come in two main forms:
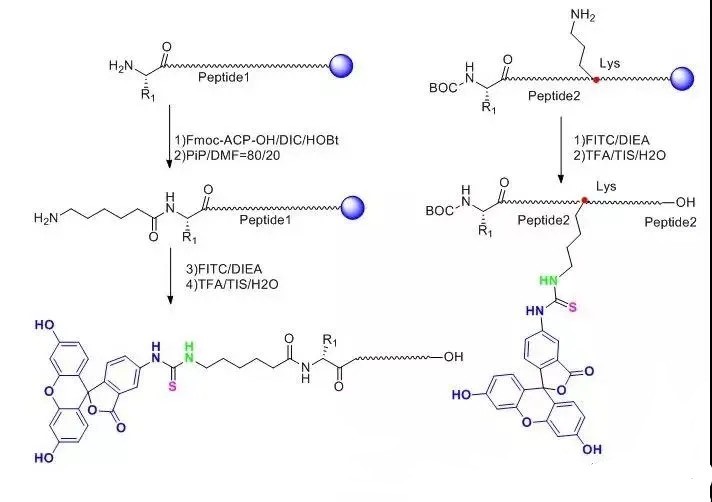
(1) FITC is inserted at the N end of the entire peptide chain, and a molecule of Acp (6-aminocaproic acid) is inserted before the FITC, also known as the alkyl spacer.
In the reaction, FITC reacts with the exposed -NH2 on the peptide chain, and the access of Acp provides a straight chain space of six carbons, which greatly reduces the steric hindrance of the reaction, improves the reaction efficiency and reduces the difficulty of the reaction.
Secondly, FITC also reacts with -SH and side chain -NH2 in the peptide structure, and the addition of Acp also reduces the possibility of such side reactions.
In addition, when the peptide is cut under acidic conditions, the peptide connected to FITC at the n-terminal needs to undergo cyclization to form luciferin, which is usually accompanied by the removal of the last amino acid, and the addition of the alkyl spacer Acp avoids this situation.
(2) One of the Lys side chains in the whole peptide is linked to FITC, and the Lys side chain is a four-carbon straight chain alkyl with the end of -NH2, which directly plays a role in reducing steric hindrance.
This modification method can flexibly modify FITC anywhere in the entire peptide, not just at the end.
4.2 AMC Modification
7-amino-4-methyl coumarin (AMC) is a widely used fluorescent labeling reagent, such as enzyme trace determination, enzyme identification, laser dye preparation, etc. In addition, C-terminal coumarin-modified ubiquitin molecules are also important probes to study protein ubiquitination process.
Different from other fluorescent dyes, AMC modified peptide molecules are carried out from the C-terminal:
(1) AMC reacts with the first amino acid at the C-terminal of the peptide chain;
(1) The whole peptide chain (starting from the second amino acid) is synthesized in solid phase, and the side chain protection group and the last amino protection group of the whole peptide chain are retained;
(2) Liquid phase condensation of AA-AMC with fully protected peptide chains;
(4) The protective group is removed to complete the modification of the peptide chain.
4.3 5-FAM modification
It is commonly used in confocal laser microscopy and flow cytometry applications.
4.4 Rhodamine B modification
Rhodamine B is one of many Rhodamine dyes used for fluorescence determination.
4.5 5-TAMRA modification
5-TAMRA is one of the most popular orange fluorophores used to label peptides and proteins.
4.6 Cy 3 and Cy 5 modifications
Cy3 and Cy5 are dyes with high extinction coefficients. Therefore, they are particularly suitable for intracellular sensitive peptide localization experiments. Their disadvantage is that they are very unstable in the peptide synthesis environment, so the amount synthesized is relatively low.
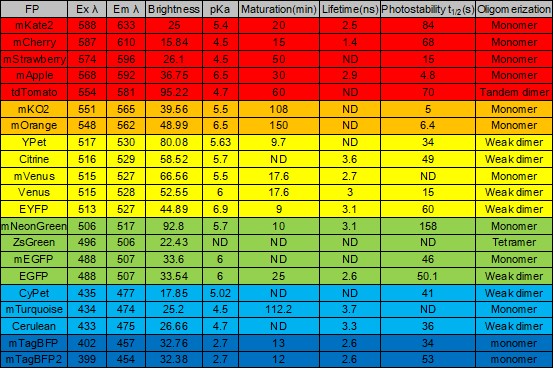
(General fluorescent protein parameter table)
In general, biotin, FITC and other dyes can be arbitrarily labeled on the N-terminal or C-terminal of the peptide. However, we recommend n-terminal tagging, which is more successful, takes less time, and is easier to operate.
Since peptides are synthesized from C to N, the n-terminal modification will be the last step of solid phase synthesis, and no additional coupling step is required. In contrast, if it is a C-terminal tag, additional steps are required, and the process is more complicated.
Most dyes are large aromatic molecules, and the involvement of such large molecules helps avoid interactions between labels and peptides. This will help maintain the conformation and biological activity of the peptide.
In general, it is recommended that the introduction of a flexible interval such as Ahx(a 6-carbon compound) makes the fluorescent labeling more stable. Otherwise, FITC will easily link to the sulfhydryl group of cysteine or the amino group of lysine at any other location.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap