Biotinized peptide modification
The very stable, non-covalent interaction between avidin and biotin is one of the most commonly used tools in chemistry and biology. Biotin is a component of complex vitamin B2. It has high affinity and binding power with chicken albumin, avidin, fungal protein and streptavidin. Both avidin and streptavidin are tetramer proteins that bind tightly to four D-biotin molecules with the strongest known non-covalent binding force in nature (Kd = 10-15 M).
The interaction between biotin and avidin can be used for protein purification, detection, solidification, drug guidance, protein structure analysis, etc.
Peptide and protein interactions
The simplest way to detect substances that interact with a peptide is to use the peptide as a affinity and pull-down test, and then directly detect the binding protein. Pull-down tests can be used to verify the presence of protein-protein interactions (predicted by other research methods, such as co-immunoprecipitation) and as a primary screening test to identify unknown protein-protein interactions. Synthetic peptides are often used to validate hypothesized protein-protein interactions by competitively blocking binding.
Biotin-labeled peptides contain a special functional domain and equivalent reference unmodified peptide, which can be anchored to avidin-conjugated magnetic beads, which are incubated with the target sample (such as nuclear extracts or purified recombinant proteins), washed to remove the unbound proteins, and then eluted the bound proteins. SDS/ PAGE analysis It can be observed directly by protein staining. By comparing the proteins that bind to the modified and unmodified peptides respectively, it is possible to identify the candidate, the "reader" protein of the specific functional protein.
Biotin-labeled peptides with some special modifications can be chemically synthesized with a purity of more than 80%. The peptide length should be 15-20 amino acids. When the target modification is in the middle of the sequence, the two amino acid residues must not be less than 6-8. In general, biotin is mostly coupled to the n-terminal or C-terminal. If there are no special requirements, we recommend biotin coupling to the N-terminal, which has the advantages of higher success rate of modification, shorter production time, and easy operation. In addition, if coupled to the C-terminal, an additional lysine is required.
Pull-down assay of biotin-labeled peptide
Biotin-labeled peptides can be conjugated with avidin magnetic beads to form resins for peptide pulldown tests. The biotin-labeled peptide was first loaded onto Immobilized streptavidin beads (Pierce) anchored with avidin, and then incubated with cell lysate. Cell lysis at 1% (v/v) Nonidet P-40, 150 mM sodium chloride, 50 mM Tris-HCl, pH 7.5, protease inhibitors (Complete Tablets, Roche Applied Science), adding 1 mM sodium orthovanadate as a phosphatase inhibitor. The same amount of protein was incubated with the anchored peptide at 4℃ for 6 hours. After extensive washing and boiling in the SDS sample buffer, the binding protein can be eluted from the anchored polypeptide or the peptide-protein complex can be disconnected from the magnetic bead with 50 mM dithiothreitol. Eluents from the active peptide and pull-downs of the control target peptide were combined for further analysis.
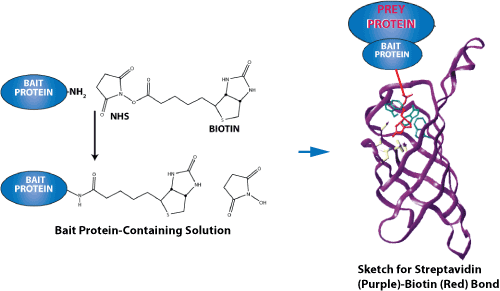
The above diagram shows the measurement of streptavidin-biotin bond strength. Due to their unusually high affinity binding, the most prominent of these ligand-receptor pairs are streptavidin-biotin and avidin-biotin. Both proteins have a tetramer structure, which allows them to bind four ligands.
Peptide modification includes biotinylation:, which is a post-translational modification(from custom peptide synthesis).Biotin can be strongly bound with avidin or streptavidin, and the binding strength is even close to covalent bond. Biotin-labeled peptides are commonly used in immunoassays, histocytochemistry, and fluorescence-based flow cytometry. Labeled anti-biotin antibodies can also be used to bind biotinylated peptides. Biotin labels are often attached to the lysine side chain or N-terminal. 6-aminocaproic acid is often used as a bond between the peptide and biotin, which allows the bond to bind the substrate flexibly and better in the presence of steric hindrance.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap