Amino protective reagent: alkoxycarbonyl type
Peptide synthesis is constantly improving, from small-scale, short peptide chain synthesis to large-scale, long peptide chain synthesis. The anti AIDS drug T-20, which was launched in 2003, is a small peptide drug composed of 36 amino acid residues. In the 1990s, after the combination of combinatorial chemistry and peptide synthesis technology, the synthesis of combinatorial peptide library appeared, which played a great role in the fields of chemistry, biochemistry, medicine, molecular biology and so on. In the process of development, the requirements for the protection of amino acids have also been continuously improved.
Requirements for peptide synthesis:
In solid-phase peptide synthesis, it is required that the raw amino acids participating in the reaction do not participate in the formation of peptide bonds, and the active functional groups are protected. At the same time, after the reaction, the protective groups of these active groups can be removed
Principles for selecting protective reagents
When selecting amino acid protective groups, the problem of reaction selectivity should be fully considered, that is α- Amino protective groups and α- Carboxyl protective group is a temporary protective group. After an amino acid is bound to the peptide chain, it should be removed immediately; The side chain protective group is a "permanent" protective group, which will not be affected by the reactant during the synthesis of the peptide, and will not react. It will be finally removed after the peptide synthesis. Therefore, the stability of the side chain protective group is greater than α- Amino protective groups and α- Carboxyl protecting group.
The selection of protective amino acids is directly related to the yield and purity of synthetic products. The by-products formed in the reaction and racemization bring great difficulties to purification. Therefore, the development of new protective amino acids is an important part of solid-phase peptide synthesis. At present, amino acid protection is mainly divided into three categories: α- Amino protection α- Carboxyl protection and side chain active group protection.
Amino protection (alkoxycarbonyl type)
Amino groups can be reversibly shielded by acylation, alkylation, alkylacylation and other reactions. Protective groups based on sulfur and phosphorus derivatives have also been reported. Over the past 10 years, hundreds of different amino protective groups have been developed, mainly including alkoxycarbonyl protective groups, acyl protective groups and alkyl protective groups. The results show that there is no universal ideal amino protective group for peptide solid-phase synthesis.
Common group protectants: Fmoc-OSu, CBZ-OSu
Z. Boc and Fmoc are the three most common amino protective groups. Z is an amino protecting group that has been used for a long time and is still widely used. Its advantages are easy preparation: the obtained Z - amino acid is easy to crystallize and stable; It is not easy to racemate when activated; The protective group can be removed under the conditions of h2/ PD, hbr/ AcOH, na/ liquid ammonia, etc. BOC can be used in the coupling process of most peptide synthesis, which can be removed under acid hydrolysis conditions, while avoiding the effects of catalytic hydrogenation, alkali hydrolysis and sodium / liquid ammonia reduction. B is usually removed under anhydrous TF and ℃ reaction conditions. Fmoc is the only widely used amino acid protection group of carbamate type that can be dissociated under weak base conditions. Fmoc deprotection can be completed at room temperature with dilute piperidine solution or diethylamine DMF solution
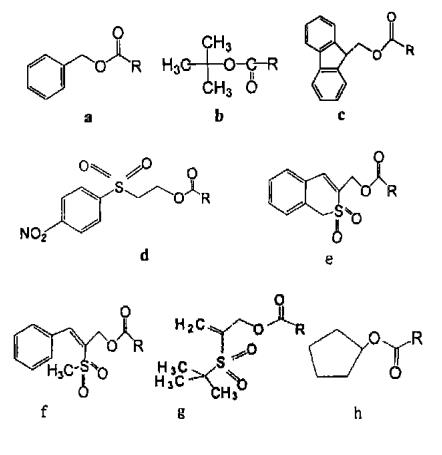
a :Cbz(Z) b :Boc
c :Fmoc ; d :Nsc
e :Bsmoc ; f :Mspoc
g:Bspoc ; h:Poc
NSC is considered to be a better protective group than Fmoc. NSC is more stable under alkaline conditions and less sensitive to weak alkaline solvents. NSC is more hydrophilic than Fmoc, and the possibility of forming asparagine is further reduced
POC is an amino protective group different from BOC and Fmoc. It is stable to both acid and alkali. Surajit et al. Found that when reacting with trifluoroacetic acid at 25 ℃ for 1 h, POC group is still very stable, while side chain protective groups such as tert butyl can be completely removed. Using this feature, side chain protective groups can be selectively removed. POC groups can be removed with tetramolybdate under ultrasonic agitation, and can also be removed with HBr/ AcOH or na/ liquid ammonia.
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap