Peptide Synthesis Service India

Peptide synthesis is the process of connecting various amino acid units in various orders and connection methods according to the customer's research and development needs. Due to the fact that amino acids exist in the form of intramolecular zwitterions under neutral conditions, it is difficult to directly condense amide acid reactions between amino acids under normal conditions.
1. Popular API peptides and impurities targeting the Indian market: Semaglutide, Exenatide, Salmon Calcitonin, etc
2. More than 300 types of peptide modifications, disulfide bonds, peptide cyclization, phosphorylation, fluorescence modification, etc.
3. Popular Fmoc/boc amino acids, unnatural amino acids, peptide reagents such as Fmoc-OSu are available in stock.

Custom Peptide Synthesis Peptide Impurities Synthesis

Fmoc/boc amino acids Peptide Reagents
About Omizzur Peptide
Omizzur is a biotech company headquartered in the United States, engaged in peptide synthesis and amino acid synthesis for over 5 years. At present, there is a significant price advantage in peptide impurities, fmoc amino acids, and peptide reagents.
Contact the Omizzur team to obtain the latest product catalog and price list.
FAQs of Peptide Synthesis
Peptide synthesis involves first connecting the hydroxyl group of the amino acid at the hydroxyl end of the peptide chain to an insoluble polymer resin in a covalent bond structure. Then, the amino acid bound to the solid-phase carrier is used as an amino component to remove the amino protection group and react with excess activated carboxyl group components, elongate the peptide chain, repeat the operation, and reach the desired peptide chain length. Finally, the peptide chain is cleaved from the resin and purified to obtain the desired peptide.
Among α- The use of BOC protection for amino groups is called BOC solid-phase synthesis method, α- The use of FMOC protection for amino groups is called FMOC solid-phase synthesis method.
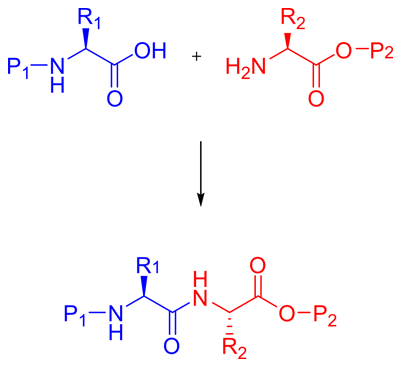
1. Peptide Synthesis Method - Omizzur
Solution-phase synthesis
Liquid phase peptide synthesis is a classic method for peptide synthesis. Although most of them have been replaced by solid-phase peptide synthesis in the laboratory, liquid-phase peptide synthesis still has the potential for large-scale industrial production of peptides.
Solid Phase Peptide Synthesis
Solid phase peptide synthesis (SPPS), pioneered by Robert Bruce Merrifield, has become a recognized organic synthesis method for producing peptides and proteins in the laboratory. Solid phase peptide synthesis can synthesize natural peptides that are difficult to express in bacteria, add non natural amino acids, modify peptide/protein skeletons, and synthesize proteins with a D-configuration.
2. Solid Phase Peptide Synthesis Process
Generally speaking, the process of solid-phase peptide synthesis is as follows: condensation washing removal protection washing in one cycle; The free N-terminus and N-terminus are condensed by amino acids with exposed carboxyl groups, and then the protection is removed, exposing the new N-terminus again to continue the reaction.
The most important factor to consider in solid-phase synthesis is the yield of each step. If the yield of each step is not high, the overall reaction yield will become extremely poor. Therefore, each amino acid must be added in excess (two to ten times) to optimize the overall reaction yield. Two commonly used solid-phase peptide synthesis methods are Fmoc and Boc. Solid phase peptide synthesis is different from ribosomal protein synthesis, with a direction of synthesis from the C-terminus to the N-terminus, where the N-terminus amino acid monomer is protected by the unprotected amino acid monomer of the peptide added with Fmoc and Boc groups.
Automated synthesizers can also be used for two technologies, but many research groups continue to manually perform solid-phase peptide synthesis.
3.Advantages of Solid-Phase Peptide Synthesis
Solid phase peptide synthesis has been launched for over 40 years, and there have been significant technological optimizations: firstly, the use of resin based optimizations. Secondly, there is an improvement in the attachment and cleavage of the linker between the C-terminal amino acid and the polystyrene resin at a roughly quantitative yield. Thirdly, the evolution of side chain protection limits the side chain reactions of non target substances. Fourthly, there is an improvement in the newly activated carboxyl groups of the input amino acids, improving coupling and reducing epimers. Finally, the process itself has been optimized.
When Merifield first reported, he took off his protection α- Amino groups lead to the formation of complex salts between peptides and resins, requiring alkaline neutralization before coupling. In addition, when the amino acid monomer of a peptide binds to the next amino acid monomer, the peptide may aggregate and form a secondary structure, which has an adverse effect on coupling. Kent et al.'s study showed that neutralization α- The coupling of amino acids with the next amino acid can improve condensation.
4. Problems in Solid-phase Synthesis
The yield of solid-phase peptide synthesis is limited, and the produced peptides or proteins are only limited to 70 amino acid monomers. The difficulty of synthesis belongs to sequence dependence, and it is also difficult to synthesize typical amyloid like peptides and proteins. If longer peptides are to be synthesized, they must be connected through natural chemical connections between two segments of peptides in a quantitative yield.
5. Peptide Modification:
There are many types of peptide modifications, which can be simply divided into post modification and process modification (using derived amino acid modifications). From different modification sites, they can be divided into N-terminal modification, C-terminal modification, side chain modification, amino acid modification, skeleton modification, etc. As an important means of altering the main chain structure or side chain groups of peptide chains, peptide modification can effectively alter the physicochemical properties of peptide compounds, increase water solubility, prolong the in vivo action time, alter their biological distribution, eliminate immunogenicity, and reduce toxic side effects.
6. Peptide Cyclization
Cyclopeptides have many applications in biomedical fields, and many naturally occurring peptides with biological activity are cyclic peptides. Due to the fact that cyclic peptides are often more rigid than linear peptides, they have extremely strong resistance to the digestive system, can survive in the digestive tract, and exhibit stronger affinity for target receptors. Cyclization is the most direct way to synthesize cyclic peptides, especially for peptides with larger structural frameworks.
7. Side chains: disulfide bonds
The most common type of side chain side chain cyclization is disulfide bridging between cysteine residues, which is introduced by deprotecting a pair of cysteine residues and then oxidizing them to form disulfide bonds. Polycyclic synthesis can be achieved by selectively removing thiol protecting groups. Cyclization can be completed either in the solvent after dissociation or on the resin before dissociation. Due to the difficulty of forming cyclizable conformations of peptides on resins, cyclization on resins may be less efficient than in solvents.
8. Peptide Phosphorylation
Phosphorylation is one of the most common post-translational modifications in nature. In human cells, over 30% of proteins are phosphorylated. Phosphorylation, especially reversible phosphorylation, plays an important role in controlling many cellular processes, such as signal transduction, gene expression, cell cycle and cytoskeleton regulation, and cell apoptosis.
Phosphorylation can be observed on various amino acid residues, but the most common phosphorylation targets are serine, threonine, and tyrosine residues. Phosphate tyrosine, phosphothreonine, and phosphoserine derivatives can be introduced into peptides during synthesis or formed after peptide synthesis. Selective phosphorylation can be achieved by using serine, threonine, and tyrosine residues that can selectively remove protective groups. Some phosphorylation reagents can also introduce phosphate groups into peptides through post modification.
 Quote & Order:
Quote & Order:
1. Click to send inqauiry online: Make Inquiry Now
2. Send mail to us: [email protected]
* Please mail us your product name and quantity needed, Omizzur will get back to you within 1 hour.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap