The Origin and Development of Peptide
Abstract:Because the synthesis of peptide drugs is simpler than protein, there is no quaternary structure, almost all amino acids after degradation, easy to absorb. The research and development time of peptide drugs is shorter than that of protein drugs, and the side effects of peptide drugs are relatively small, so the research of peptide drugs is developing rapidly at home and abroad.
Peptide drugs are mostly solid phase peptide synthesis methods, due to the side chain protection group, coupled groups, enolized, the intermediate transition state is vulnerable to external environmental influence and instability, and easy to be oxidized, easy to produce impurities, and production, by the solvent, protection group, pressure influence, produce derivatives or produce one or two groups are oxidized, catalyzed groups.
These substances that are very similar in structure to peptide drugs are not easy to separate, and the substances that are changed or catalyzed may not only not have the drug value of peptides, but also may be extremely toxic. When impurities are analyzed by mass spectrometry, the main substances will cover the signal of impurities, so that the purity is much larger than it actually is. However, the actual laboratory separation technology of impurities is far from meeting the market requirements, and these impurities have not been paid attention to, which may have potential harm to the human body.
1: Origin And Development of Peptide Grugs in Vitro
Peptides are amino acid-derived compounds that contain at least one amide (peptide) bond. From a structural point of view, peptides include various types of peptides, such as linear peptides, cyclic peptides, degreasing peptides, etc., according to function can also be divided into antimicrobial peptides, hormone regulatory peptides, neuroactive peptides
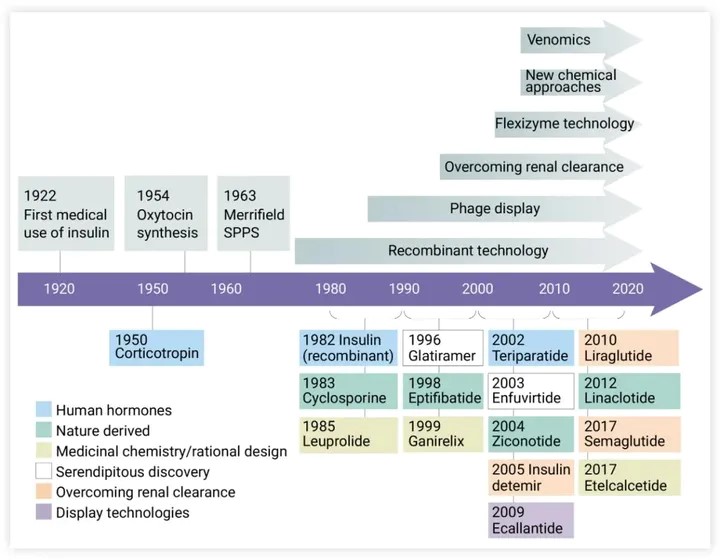
In the early 20th century, research on peptides focused on the role of human signaling hormones. Insulin is a classic example of endogenous hormone therapy. It was the first peptide drug to be used clinically and by far the most commercially successful because it revolutionized the treatment of type 1 diabetes.
Despite the success of early hormone analogues, the production of longer peptides has been limited by synthetic methods. Therefore, the selective expression of endogenous human peptides and proteins in cell culture systems is highly desirable, and the emergence of recombinant technology is a milestone in peptide drug development. In 1982, the first recombinant human peptide, somatostatin, was produced.
Subsequently, burgeoning genetic engineering enabled the adjustment of individual amino acids to improve the absorption, distribution, metabolism, and excretion characteristics of peptides in the body. Display technologies such as phage displays, as well as new chemical methods, are also advancing the field.
2: Advantages And Disadvantages of Peptide Drugs and New Attempts
he key factors for the success of peptide drugs are the effectiveness, specificity and safety of the peptide mode of action. The rapid clearance of peptides in the body means that they do not accumulate in tissues and are relatively less toxic to humans. However, the limitations of peptide drugs are as obvious as their advantages.
Because peptide drugs are easily eliminated in serum, the bioavailability of peptide drugs is low. Moreover, peptides generally have poor cell membrane permeability, which limits their use in targeting intracellular targets.
Therefore, the development of peptide therapy is mainly focused on extracellular targets. Moreover, because it cannot penetrate the intestinal mucosa and needs to be administered subcutaneously or intravenously, the convenience and compliance of peptide drugs in actual treatment are reduced
Improving the bioavailability and efficacy of peptide drugs is also a hot research area. Universal and repeatable oral administration of peptide drugs, as well as intracellular delivery, has also advanced. One category of peptide drugs, cyclic peptides, is an emerging form of drug that addresses the problem.
2.1 Cyclic peptides --A new form of peptide drugs
Cyclic peptides (including cyclic deposited peptides and bicyclic peptides) have many good properties as therapeutic drugs and research tools. Compared with linear peptides, cyclic peptides have better proteolytic resistance and structural stability. Omizzur Biotech undertakes a variety of custom synthesis of cyclic peptides, as well as custom synthesis solutions
First, cyclization of the peptide reduces the degree of conformational freedom, greatly enhancing its metabolic stability and binding affinity/specificity to the target molecule. In general, cyclic peptides with small ring size (10 aa) are relatively resistant to proteolytic degradation.
Second, intermediate-sized cyclic peptides (6-15 aa; The most commonly used is MW = 500-2000 Da), which is typically 3-5 times larger than traditional small molecule drugs (MW 500) and can form a larger surface area with the target protein. As a result, cyclic peptides have the ability to reproduce special protein affinity and specificity, even for targets without any binding pockets.
Third, cyclic peptides have great structural diversity. Using just these 20 protein-source amino acids, 208 different cyclic peptides can be produced. The structural diversity can be further increased by adding non-protein source amino acids.
Fourth, compared to proteins, cyclic peptides retain some properties of small molecules, such as stability, lower risk of immune response, synthesability, and lower production costs.
>> More infos about cyclic peptide synthesis: https://www.omizzur.com/services/specialty/cyclic-peptide/
2.2 Peptide-like - chemically synthesized peptide drugs
In the strategy of new drugs, in order to overcome the defects of peptide instability, in addition to different degrees of modification of peptides like cyclic peptides, peptide-like compounds are another reasonable means.
Peptide-like compounds are a class of compounds whose pharmacophore mimics a natural peptide or protein in three-dimensional space and retains the ability to interact with a biological target to produce the same biological effect. The difference is that the peptides avoid the inherent defects of natural peptides and improve the biological activity and stability.
3. Screening of Peptide Drugs
Peptide drugs, whether isolated from the innate immunity of various species (including mammals, amphibians, fish, insects, plants, and bacteria) or designed based on structural activity relationship studies, have great potential as a new class of structural drugs. This paper introduces a high-throughput method to quickly identify and find suitable drugs - the construction of peptide libraries.
The construction and use of peptide libraries are generally through the collection of all required peptides, the unified culture of samples, and then through the cellular or molecular level for high throughput detection.
For example, B. Guixer et al. determined the N-terminal and C-terminal forms by selecting amino acids that they needed for specific functions, and synthesized peptide libraries composed of different arrangements of selected amino acids by mixed-resolution method. Then, through experiments simulating the blood-brain barrier, they screened peptides from the peptide library that could pass, and finally achieved the ability to cross the blood-brain barrier model in vitro (shuttle peptide).
Currently, there are nearly 100 peptide drugs on the global market, and research on new peptide therapeutics continues at a steady pace, with more than 100 peptides in clinical development and another 400-600 peptides in preclinical research. The use of peptides as therapeutics has evolved over time and continues to evolve as drug development and treatment modalities change.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap