synthesis and purification of short peptide raw materials
Starting material for peptides
A peptide starting material is defined as a raw material, intermediate, or product used to produce an API that is incorporated into the API structure as an important structural fragment, usually a protected amino acid derivative. Current research work on raw materials in the field of peptides includes the selection of raw materials, the detection and control of impurities in the starting material, the tracking of impurities in the various steps of the production and preparation process (removal capacity and residues), and the relationship between impurities in the raw material and impurities in the final API.
The traditional preparation of peptide starting materials is often through column chromatography separation or preparative separation, which greatly limits the production scale of raw materials, and both separation methods consume a lot of time and reagents. How to produce high quality, low cost, safe and stable starting raw materials has become an urgent problem for many companies to solve.Omizzur aims to provide customers with quality and quantity API products, so it pays more attention to the quality of API starting materials. In the process of studying the production of peptide starting raw materials, we found that some specific short peptide raw materials have a crystalline structure, and such short peptides with a crystalline structure can be controlled by crystallization (See Figure 1), so as to achieve the purpose of low-cost and high-quality production.
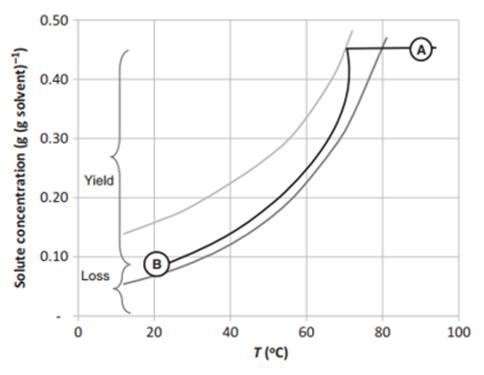
Figure 1. Solution trajectory during batch cooling crystallization in aqueous solutionSolubility (dark gray line) and metastable limit (light gray line)
Crystallization of short peptides
For the crystallization of short peptides, first of all, it is necessary to determine whether the short peptide has a crystal form, which requires the detection of XRPD and DSC of the product (See Figure 2, 3). The laboratory has observed whether the short peptide can become a solid with good fluidity and uniform dispersion in solution as the basis for judgment. Second, the short peptide needs to be tested for solubility in different solvents, and the good solvent and bad solvent that the short peptide can be used are selected. Thirdly, the short peptide was mixed with different proportions of solvents to determine the proportion of mixed solvents to ensure the purity and yield of short peptide. Finally, the kinetic factors such as temperature, crystallization point and aging time of the short peptide were confirmed by simulating the production conditions.
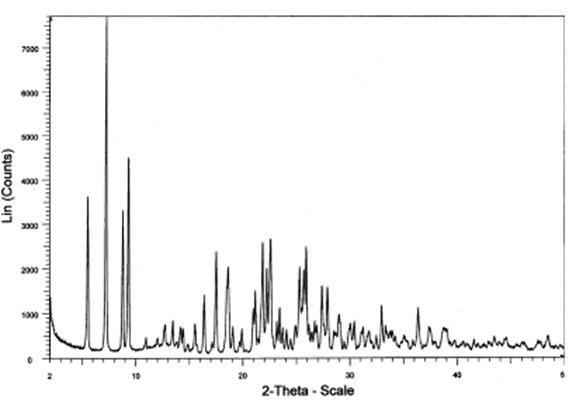

Figure 2. XRPD data
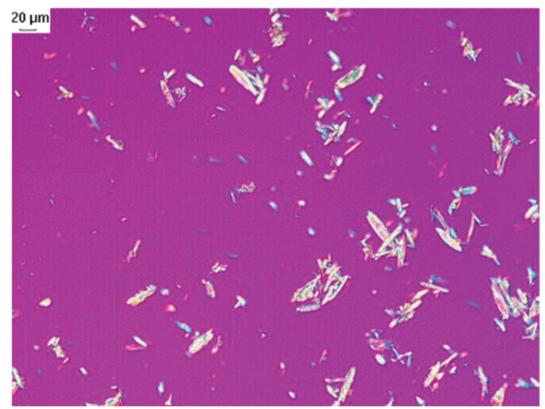
Figure 3.Photomicrograph data
Preparation of short peptides
For the preparation of short peptides, the reaction is relatively single (condensation reaction and deprotection reaction). Among them, DMF was selected as the solvent of condensation reaction, and most of the reactions involved in this solvent were separated by pouring DMF reaction liquid into water to precipitate short peptides. The disadvantage is that in the water evolution process, all insoluble compounds are precipitated at the same time, making the product contain various impurities, limiting the purity of short peptides. Combining the characteristics of traditional crystallization method and short peptide reaction, Omizzur uses crystallization method to complete the reaction, quenching, separation and purification of short peptide production in the same reactor. With years of practice and accumulation of international peptide related industry technologies, Omzzur has fully demonstrated the professionalism and leadership of the company's team in the whole process of peptide synthesis new peptide drug research and development, apis, preparations, quality control, registration, and international cooperation.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap