Study on long-acting sustained-release microspheres of exenatide
Exenatide, a glucagon-like peptide GLP-1 receptor agonist, is used to improve glycemic control in patients with type 2 diabetes. In order to improve patients' medication compliance and reduce adverse drug reactions,
Exenatide, as the first incretin-like drug used in clinical practice, has a significant hypoglycemic effect, and will stop acting after the blood sugar returns to normal, without causing hypoglycemia.(for exenatide impurities pls visit) However, since the half-life of 1 is several hours, it needs to be injected twice a day, the patient's compliance is poor, and adverse reactions have been found in a considerable proportion of patients, so the preparation of 1's long-acting sustained-release preparation has clinical advantages.
In 2017, AstraZeneca announced that the U.S. FDA approved Bydureon BCise (extended release) injection as an add-on therapy to basal insulin for adults with type 2 diabetes who have insufficient glycemic control. This does not solve the phenomenon of dosing delay, nor does it eliminate the adverse reactions of the drug. Therefore, the design and development of 1 microsphere with a reasonable drug release curve, no adverse drug reactions, and long-acting sustained release for 1 month has become the focus of research.
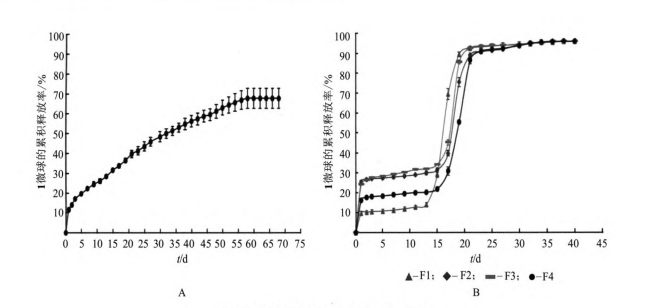
In the experiment, the membrane emulsification sedimentation method was used to prepare 1 long-acting slow-release microspheres. The obtained microspheres were spherical, with a smooth surface, good particle size uniformity, and an encapsulation rate of up to 90%. The in vitro release curve showed that the 30-day cumulative release rate of 1 microsphere was close to 100%. Magnesium hydroxide can neutralize the acidic substances produced by the degradation of PLGA, inhibit the autocatalytic degradation of PLGA, and delay the release rate of the drug. In addition, the release rate of microspheres can be adjusted by adding dextran, and the release rate of 1 microsphere can be appropriately increased, so as to reach the minimum therapeutic concentration during the release period, and the cumulative release rate is close to 100% at the end of the release. This method is simple to operate and easy to prepare on a large scale. 1 microspheres have bright application prospects in the clinical treatment of type 2 diabetes. The next step should be to further optimize the prescription, and conduct in vivo pharmacodynamic and pharmacokinetic tests to investigate the release of 1 microspheres in vivo.
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap