Multifunctional chimeric peptide entry strategy
A novel chimeric peptide was proposed
Xia Biolecular Research and Development team of Xiamen University proposed a novel chimeric peptide biolecular delivery system and successfully used it in vivo treatment model of acute liver injury in mice.
This study not only provides a new strategy for intracellular delivery of biomacromolecules, but also lays a foundation for basic research and clinical application of protein drugs targeting intracellular targets. This is a big step forward in peptide synthesis.
The occurrence and development of diseases are usually related to the abnormality of intracellular signal network, and the intervention by using drugs to target and regulate the corresponding key molecules or functional pathways is an important means of basic research and clinical treatment.
Biomacromolecule drugs have no restrictions on the target, and have unique advantages in specificity and efficacy, so they have become one of the most promising and competitive fields in drug development, but biomacromolecules are difficult to freely penetrate the cell membrane into the cell, so the targets of the currently approved biomacromolecule drugs are mainly located outside the cell membrane or on the cell membrane. However, the vast majority (70%) of potential drug targets are located inside the cell, so how to effectively deliver biomacromolecules into the cell has become an urgent task to explore this unknown treasure trove of drugs.
Cellular transmembrane peptides can mediate the entry of biomacromolecules into the cell
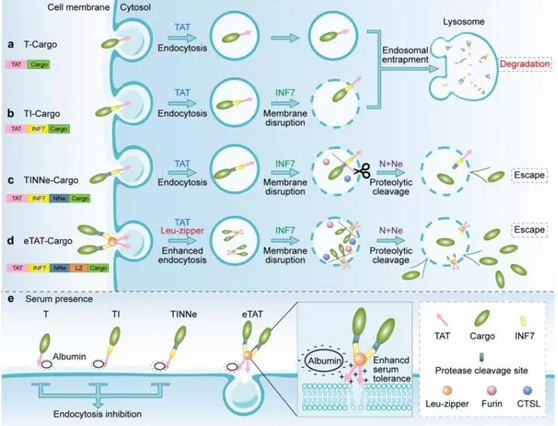
Cell-penetrating peptides (CPPs), such as TAT, can mediate macromolecules into the Cell, and have the advantages of high biocompatibility, easy operation, low toxicity and side effects, so it is one of the research focuses in the field of protein delivery.
On the basis of TAT, the research team also introduced PH-sensitive peptides, endocytosomal vesicle specific protease cleavage sites and polymerization domains, overcoming the technical bottlenecks in the application of CPPs above and constructing a Mosaic peptide delivery system eTAT (enhanced TAT) (Figure 1d). The cleavage of the enzyme cleavage site in the system by proteinase in the endocytosis vesicles was utilized to facilitate the separation of cargo molecules from the delivery system, thus achieving effective endocytosis vesicles escape (FIG. 1c). The addition of polymerizing domains, such as leucine zippers, overcomes the effect of serum on delivery efficiency (Figure 1e). Subsequently, the research team took RIP3 protein, which mediates the cellular necrosis pathway, as the drug target, and fused the eTAT system with the functional protein Ppm1b to form the therapeutic molecule ETAT-PPM1b for the therapeutic study of acute liver injury caused by APAP (acetaminophen, Tylenol ®).
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap