Disulfide bond problems in peptides
Importance of disulfide bonds in peptide synthesis
Disulfide bonds are an integral part of the three-dimensional structure of many proteins. We can find these covalent bonds in almost all extracellular peptide and protein molecules.
A disulfide bond is formed when the sulfur atom of a cystine forms a covalent single bond with the sulfur atom of the other half of the cystine at a different location in the protein. These bonds help stabilize proteins, especially those secreted from cells.
The effective formation of a disulfide bond involves many aspects, including proper management of cysteine, protection of the amino acid residues, removal of protective groups and pairing methods.
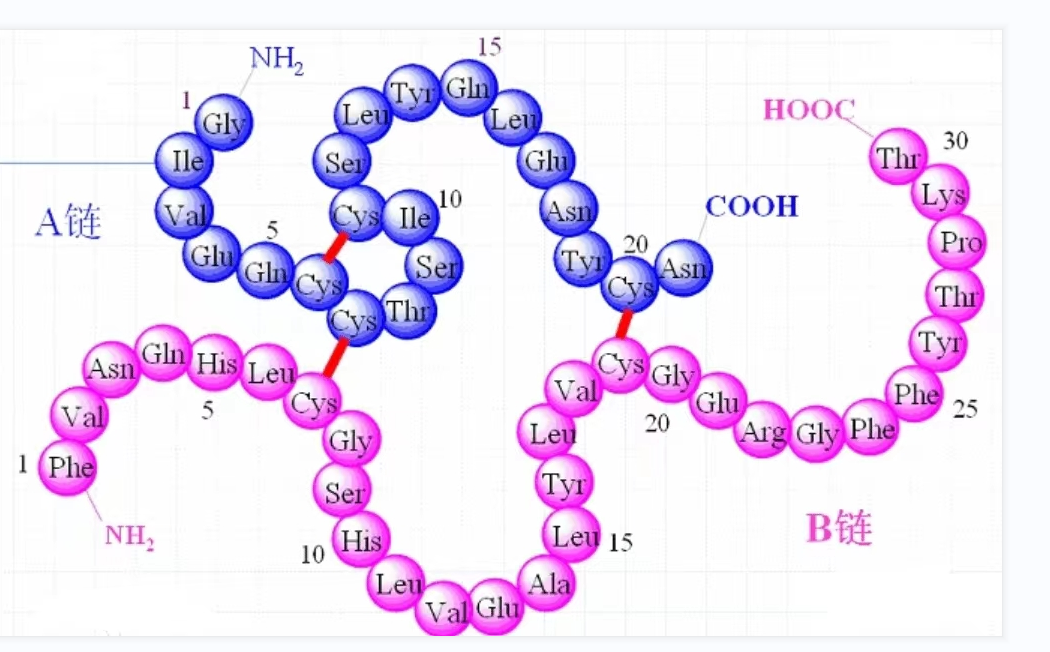
Disulfide ring - forming technique is very important in peptide synthesis.Omizzur Biotech have developed disulfide ring formation techniques. If the peptide contains only one pair of Cys, the disulfide bond formation is simple. The peptides are synthesized in either solid or liquid phase and then oxidized in solution pH8-9. When two or more pairs of disulfide bonds need to be formed, the synthesis process is relatively complex. Although disulfide bond formation is usually accomplished at a later stage of the synthesis regimen, sometimes the introduction of preformed disulfide is beneficial for bonding or lengthening the peptide chain.
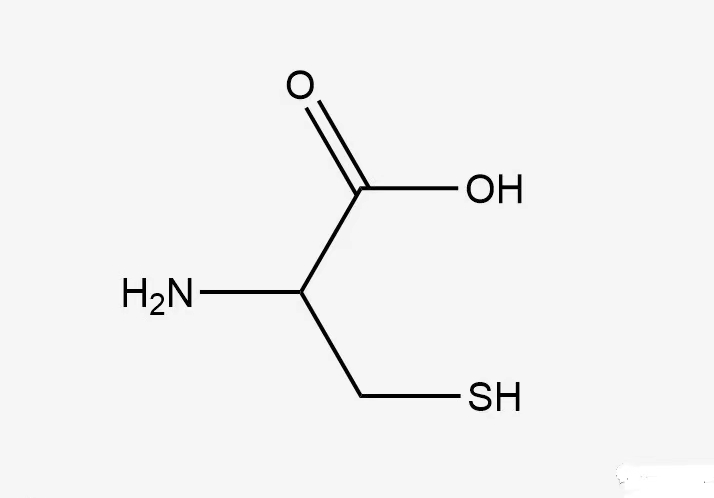
Why is cysteamine (Cys) so special?
The side chain of Cys has a very active reactive sulfhydryl group. The hydrogen atoms in this group can be easily replaced by free radicals and other groups, and thus easily form covalent bonds with other molecules.
Disulfide bonds are an important part of the three-dimensional structure of many proteins. Disulfide bridge bonds can reduce the elasticity of the peptide, increase stiffness, and reduce the number of potential conformations. Such conformation constraints are essential for biological activity and structural stability.Hydrophobic amino acids such as Leu, Ile, Val are the stabilizer of the helix. Because even if cysteine doesn't form a disulfide bond it can stabilize the alpha helix. That is, if all cysteine residues are in the reduced state (-SH, carrying free sulfides), a high percentage of helical fragments will be possible.
Disulfide bonds formed by cysteine are permanent for tertiary structure stability. In most cases, S-S bridge pairs between bonds are necessary to form quaternary structures. Sometimes the cysteine residues that form disulfide bonds are far apart in the first-order structure. The topology of disulfide bond is the basis of analyzing the homology of protein primary structure. Cysteine residues located in homologous proteins are very conserved. Tryptophan alone is statistically more conserved than cysteine
Cysteine is located in the center of the thioglycolase catalyzed site. Cysteine can form acyl intermediates directly with the substrate. The reduced form acts as a "thiol buffer" to maintain the cysteine in the reduced state of the protein. At low pH, the equilibrium favors the reduced state, the -SH form, whereas in alkaline environments, -SH is more likely to be oxidized to form -SR, where R is anything but hydrogen.
Cysteine can also be used as detoxics to react with hydrogen peroxide and organic peroxides.
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap