Design and synthesis of antimicrobial peptides
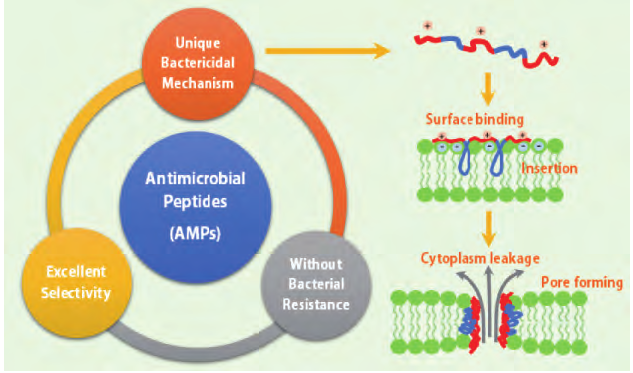
Antimicrobial peptides are cationic short peptides in most organisms, which constitute an important part of biological immune system. Antimicrobial peptides have broad-spectrum antibacterial activity and cell selectivity. Their unique mechanism of membrane destruction and sterilization is not easy to cause drug resistance mutation of pathogens. They are expected to become a new generation of effective "antibiotics" to control pathogens. However, the extraction cost of natural antimicrobial peptides is high, the yield is low, and the cycle is long, which is not conducive to large-scale production and promotion. Therefore, the synthesis of antimicrobial peptides and their simulated polymers based on chemical synthesis came into being. This method provides unlimited possibilities for the design and synthesis of antimicrobial peptides.
The source of antimicrobial peptides
The first natural antimicrobial peptide found was thionin from plants. Until the 1980s, Swedish scientist Boman and others reported that more and more bioactive antimicrobial peptides were isolated from microorganisms, amphibians, plants, mammals and even humans. At present, there are 2900 kinds of antimicrobial peptides from different organisms. Based on the database of antimicrobial peptides in 2013, the distribution of antimicrobial peptides was analyzed. Animals (76%), plants (13%) and bacteria (1.8%) were the main sources of natural antimicrobial peptides.
Synthesis of antimicrobial peptides
In the early stage, the natural antimicrobial peptides were extracted and separated directly from the organism by researchers. However, this method is relatively difficult, the yield is low, the technical requirements are high, and the cost is high, so it can not be applied to the large-scale production of antimicrobial peptides. The researchers mainly synthesized antibacterial peptides with specific amino acid sequences by condensation reaction of amino acids. In the process of synthesis, the functional groups of amino acids were used to protect and protect them to achieve the goal of directed synthesis. The chemical synthesis of polypeptide can be divided into two kinds: liquid phase synthesis and solid-phase synthesis according to whether solid-phase carrier is used in the synthesis process. Amino acids or short peptides react in solution in liquid synthesis. In the process of synthesis, intermediate products need to be purified, which is inefficient and difficult. Therefore, it is less used in the synthesis of antimicrobial peptides.
The synthesis of antimicrobial peptides is mainly by solid-phase synthesis, mainly through the chemical groups on the solid resin carrier connected with the carboxyl end of amino acids, then the amino acid protection group is removed, the next amino acid is connected, through repeating the above steps, the sequence of peptides is obtained, and finally, the peptide is removed from the resin with corresponding chemical reagents. Compared with liquid phase synthesis, solid phase synthesis is convenient and rapid, easy to purify and the sequence structure of antibacterial peptide is precisely controlled.
In the 1940s, a method of synthesis of polypeptides by N-carboxyanhydrides (NCA) was developed. NCA ring opening polymerization induced the monomer to open the ring by amino as nucleophilic reagent to obtain the polyaamino acid products with various structures such as homopolymerization, random copolymerization, grafting copolymerization and block copolymerization. NCA ring opening polymerization has the advantages of low production cost and high yield, and it can be used to synthesize high molecular weight polypeptide more easily. However, the molecular weight distribution of polypeptide prepared by this method is wide, and if two or more monomers are copolymerized, their structure is heterogeneous, so it is difficult to study the relationship between their structure and their properties. Aliferis and others have solved this problem by improving the vacuum polymerization method.
Antimicrobial peptide
The discovery of antimicrobial peptides provides a molecular framework for the development of new antimicrobial agents. The researchers pointed out that the key determinant of antimicrobial peptide activity is the presence of cationic and hydrophobic amino acid residues, not specific amino acid sequences or stable secondary structures. Based on this, scientists have innovatively proposed the concept of antimicrobial peptides, which simulate the key structural characteristics of natural antimicrobial peptides: they contain both cations and hydrophobic groups. The mechanism of antibacterial is similar to that of natural antimicrobial peptides, that is, membrane destruction mechanism, which makes it difficult for bacteria to produce resistance to antimicrobial peptides. Compared with natural antimicrobial peptides, antimicrobial like polymers have significant advantages: low cost and easy to achieve mass production, providing unlimited possibility for flexible chemical modification, and broadening the application scope of antimicrobial peptide polymers in biomedical field.
At present, the main antimicrobial peptide like polymers can be divided into two categories: one is peptide based antimicrobial polymer. Based on amino acid components, other small molecules or biocompatibility polymer components are introduced. The new component can give more excellent properties to antimicrobial peptides, such as reducing biological toxicity and drug loading.
The other is non peptide based antibacterial polymer, which can also be called polycation polymer. These polymers simulate the positive charge source of antimicrobial peptides, containing amino acids or quaternary ammonium salts (with positive charge) on the side chain of the polymer, and introducing hydrophobic copolymers into the polymer to simulate the hydrophobic amino acid residues of antimicrobial peptides
Antimicrobial peptide assembly
Zhang et al. Found the first self-assembled polypeptide in 1993. After EAK 16 (n-AEAEAKAEAEAKAK-c), a large number of studies showed that the peptide assembly has a broad application prospect in the fields of functional materials preparation, gene therapy, biosensor, tissue engineering and clinical medicine. Therefore, the self-assembly of polypeptide has gradually become a hot topic in the field of polypeptide research.
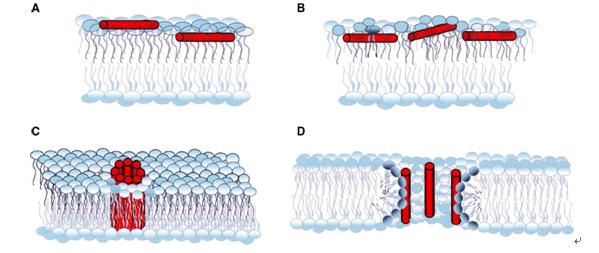
As a kind of important functional peptide, the research and application of antimicrobial peptides is of great significance to the development of biomedical. The shape of the assembly is affected by the polymer structure, hydrophobic component ratio and assembly environment, and can be assembled into vesicles, micelles, nano film structures, etc.
Antimicrobial peptides and antimicrobial like polymers are widely used as substitutes for antibiotics. Their unique antibacterial mechanism is a powerful weapon against the threat of drug-resistant bacteria. In-depth research of antimicrobial peptides will create a new era of development of new antimicrobial drugs, and have broad application prospects in animal and plant disease resistance, food antiseptic agents, cosmetics, clinical antimicrobial drugs, etc, The multifunctional assembly of antimicrobial peptides has further expanded the application range in drug encapsulation, clinical medicine, tissue engineering, biosensor and other fields.
At present, there are still many problems to be explored in the production and application of antimicrobial peptides. First of all, the molecular mechanism of antimicrobial peptides has not been clearly defined. In addition, there is a lack of research on the toxicity and structure of antimicrobial peptides. Antimicrobial peptides generally achieve antibacterial effect through membrane interaction, which will inevitably produce toxicity to normal cells. The relationship between hemolytic activity and positive charge density, hydrophobicity, amphiphilicity and helicity has been studied, which provides a theoretical basis for the rational design and optimization of highly selective antimicrobial peptides. Secondly, although antimicrobial peptides have high antibacterial activity in vitro, their antibacterial activity will be affected in vivo. Salt sensitivity of some peptides leads to their strong antagonism in normal saline, which reduces their biological activity. In addition, there are serious deficiencies in the pharmacodynamics and pharmacokinetics of antimicrobial peptides in vivo, such as the aggregation of antimicrobial peptides in vivo, the half-life in vivo, especially the sensitivity to mammalian enzymes, and the required administration frequency. The solution of these problems is of great significance for the rational design of drug delivery scheme, the minimization of biological toxicity, and the increase of antimicrobial peptide tolerance, which will also promote the clinical application of antimicrobial peptides.
The high cost of synthesis of antimicrobial peptides is still the main barrier to its wide application in clinic. There are many problems in the preparation of antimicrobial peptides, such as high cost, low yield, complex process and so on. With the deepening of antimicrobial peptide research, the demand for commercial scale antimicrobial peptide production platform is also growing. At present, the development direction is to develop short peptides with excellent effect, which is an effective way to reduce the synthesis cost.
Antimicrobial peptides suitable for clinical application should have the following characteristics: stable antibacterial activity, resistance to rapid degradation of protease in vivo, and no significant biological toxicity in complex and different environments, including human body (serum, tissue fluid, etc.). With the in-depth study on the mechanism, structure-activity relationship and pharmacodynamics of antimicrobial peptides and antimicrobial peptides, antimicrobial peptides will be greatly optimized, and will continue to play a role in the pharmaceutical industry, chemical preservatives, antibacterial coating of medical devices, especially in the pharmaceutical industry, which will become an important weapon to solve the drug resistance of traditional antibiotics and have a profound impact on human health Ring.
Read Related Articles:
Copyright © 2020 Omizzur Inc | Terms & Conditions | Privacy Notice | Sitemap